CORE PRINCIPLES
A biosimilar medicine is a biologic that is highly similar to and has no clinically meaningful differences when compared to an existing FDA-approved biological medicine, known as a reference product.
A biosimilar must be determined by the FDA to:
- be highly similar to the reference product notwithstanding minor differences in clinically inactive components and,
- have no clinically meaningful differences compared to the reference product in terms of safety, purity, and potency.
Patients and their health care providers can expect that biosimilars that are approved by the FDA will work in the same way as the corresponding reference product. Biosimilars provide patients with the same safety and efficacy.
Because all biologics have some degree of variability because they are produced in living cells, biosimilars are neither required nor expected to be “identical” to the reference biologic. Biosimilars are highly similar to the reference product, and any differences that may exist do not impact safety or effectiveness.
A detailed understanding of how the medicine works (the “mechanism of action”) can help us understand how the structure of the biologic impacts its function in the body and how changes in the structure of the biologic may affect how it works.
A biosimilar and its reference product should have the same mechanism(s) of action for each of the approved indications of use, (e.g. diabetes, breast cancer, Crohn’s disease, rheumatoid arthritis etc.), to the extent these are known
Biosimilars must meet the same product and manufacturing quality standards as any FDA-licensed biologic medicine. There are no differences in standards for reference biologics or their corresponding biosimilars.
Manufacturing biologics is complex and must be precisely controlled to obtain consistent results. This process requires state-of-the-art tools and techniques – including cell culture, protein purification, and formulation. The formulation of proteins is unique and challenging, but fill/finish is not different than other solution-based medications.
Because they are made in living organisms, all biologics, (both reference products and their biosimilars) have intrinsic batch-to-batch variability; therefore, critical quality attributes are carefully monitored at all stages of manufacture to ensure that the critical quality attributes remain within predefined ranges.
When changes are made in the manufacturing process of a biologic, the manufacturer must ensure that these changes do not impact the quality, safety and efficacy of the biologic.
The first U.S. biosimilar was approved in September 2015, and dozens of others have since been approved with many more under development.
Biosimilars have been available and used safely in Europe since 2006. In addition, biosimilars have been approved in other well-regulated regions or countries, including Australia, Canada, Japan, and Korea, expanding treatment options and access for patients and health care professionals in those regions.
A biosimilar is approved by the FDA based on head-to-head comparisons of the biosimilar and reference product. These comparisons include extensive analytical and biological function characterization, as well as human clinical comparisons, that when considered together demonstrate high similarity between the reference product and proposed biosimilar—a concept known as “totality of evidence.” The data must support a conclusion that the biosimilar is highly similar to the reference product, notwithstanding minor differences that may exist in clinically inactive components and establishes that there are no clinically meaningful differences in safety and efficacy between the biosimilar and the reference product.
In biosimilar development, the foundation of knowledge about the biosimilar begins with the collection and analysis of available public information and experience with the reference product. This includes
- Published scientific studies on how the drug works in the body (known as “the mechanism(s) of action”)
- Structural and functional aspects of the reference product,
- Peer-reviewed clinical trial and post-approval observational data on how the drug performs when administered in a real-world setting (also known as “real-world evidence”).
Published clinical trial and post-approval observational data for the reference product can be important in helping design comparative clinical studies of the reference product and a biosimilar candidate. In addition, a biosimilar sponsor will purchase multiple lots of the reference product over time in order to assess variability in the properties of the active ingredient (drug substance) and the formulation of the reference product. This publicly available information and hands-on testing of batches of reference product helps biosimilar sponsors understand the relationship between the structure of the molecule and how it functions, which together with an understanding of the mechanism of action enables the design and development of the biosimilar.
Robust analytical and pharmacological data comparing a biosimilar candidate to the reference product is a critical component in establishing the totality of evidence necessary for regulatory approval of a biosimilar. Modern analytical testing and pharmacologic assays are extremely sensitive and are generally more capable than human clinical studies of detecting a structural or functional difference between a biosimilar and reference product, should one exist. This is important because highly similar pre-clinical data between a biosimilar candidate and the reference product suggests a lower risk of observing clinical differences.
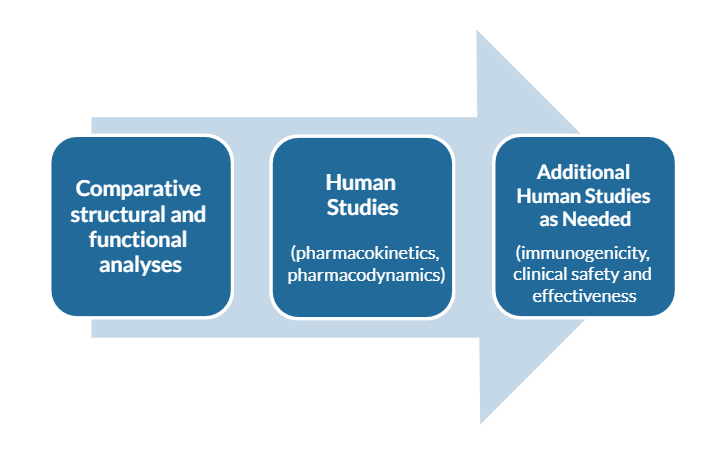
Biosimilars are approved using data obtained by the following steps. The data are evaluated at each step of development to influence subsequent steps and are used to provide extensive, head-to-head comparisons of the biosimilar and reference product at many levels, which may include:

Health authorities recommend developing biosimilars in a stepwise manner, following the following steps to provide extensive, head-to-head comparisons of the biosimilar and reference product at many levels, which may include:
-
-
- Structural and biological function comparisons, which are the foundation of biosimilarity.
- Testing of toxicity in animals, if necessary
- Human pharmacokinetic comparisons (what happens to a drug in the body)
- Human pharmacodynamic comparisons (what happens to the body in the presence of a drug)
- Comparison of the immunogenicity of the biosimilar candidate and reference product
- Human clinical studies (when necessary) to confirm that there are no clinically meaningful differences in safety and efficacy
-
The stepwise approach begins with comparative analytical testing that is conducted to demonstrate physical, chemical, and biological function similarity to the reference biologic. This includes a detailed physicochemical study of the structure of the molecule, biological function assays, and immunological properties. Additionally, an assessment is conducted to identify and quantify impurities that may be present.
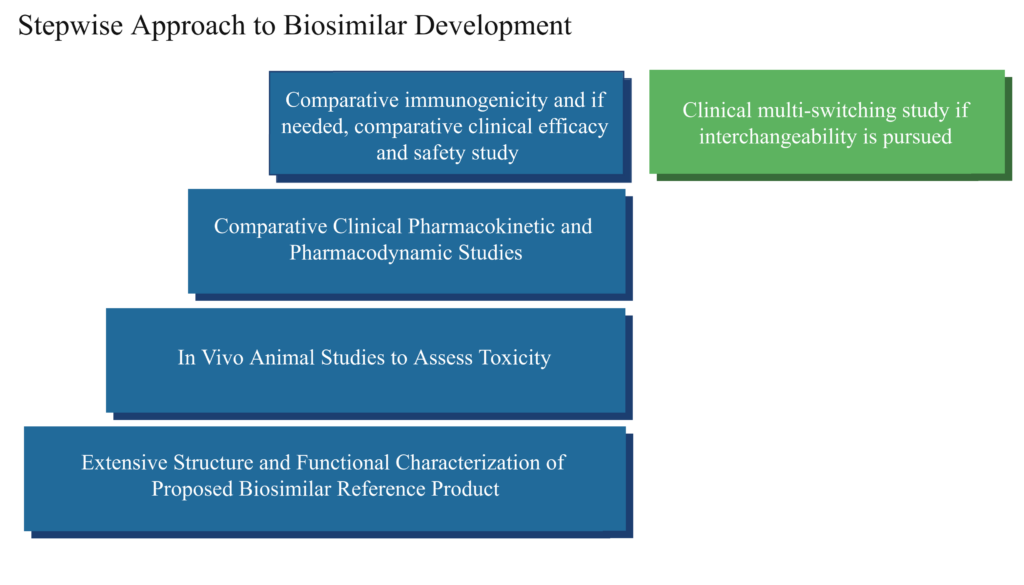
Comparative animal toxicity testing was often conducted for many early biosimilars, but over the course of years, it became apparent that there is limited useful information gathered from such studies. Nonetheless, animal toxicity studies may be conducted if there is reason to suspect that there may be toxicity differences, such as which potentially could come from use of a different cell line in the manufacturing process or if the formulation is different. “If comparative animal toxicity studies are conducted, the results obtained withe reference biologic and biosimilar candidate must be similar.
The next step is to conduct comparative pharmacokinetic (PK) and pharmacodynamic (PD) or “PK/PD” studies in humans. These studies are often conducted in healthy individuals. A PK study measures what happens to the medicine when it is in the body, including the absorption, concentration, and clearance of the medicine from the body. A PD study evaluates what happens in the body in response to the medicine by testing the effect of a medicine on a physiological function through use of a “marker,” or measurable attribute of activity or function. Comparative PK/PD studies can be conducted in healthy volunteers or patients,and are another level of evidence that helps support the fact that there are no clinically relevant differences. If there is any residual uncertainty about the similarity of the proposed biosimilar and reference product, the PK/PD studies may then be followed by confirmatory clinical studies using efficacy endpoints and safety observations. Such studies are conducted in patients with the disease being treated.
In general, the appropriate clinical trial population for a confirmatory safety and efficacy study should be uniform, should be able to detect if there is a difference in treatment response, and when possible, avoid other medications given at the same time that could complicate analysis. These studies are intended to confirm that the biosimilar works the same in patients as the reference product and to assess immunogenicity, or the comparative human immune response to the biosimilar and reference product.
Comparative immunogenicity testing of a biosimilar and reference product should be conducted in healthy volunteers or patients who have functional immune systems to ensure that a difference can be detected should one exist.
These clinical studies are not designed to re-establish the benefit and risk profiles of either the biosimilar or reference product because that information is already available from studies conducted with the reference product. Instead these studies are conducted to reduce any residual uncertainty in determining that the biosimilar is highly similar to the reference product notwithstanding minor differences that may exist in clinically inactive components, and that there are no clinically meaningful differences between the biosimilar and the reference product in terms of safety, purity, and potency.
Based on the many years of study of the reference product, along with comprehensive public information, biosimilars have structural aspects of the reference product can have an impact on clinical safety and effectiveness. The most important of these structural and functional attributes are known as “Critical Quality Attributes.” Biosimilars are developed using a hierarchy of importance of the many features of the molecular structure and manufacturing process that can be measured.
Numerous sophisticated analytical tests carefully measure and compare the structure and biological function of the reference product and biosimilar and to detect foreign or unwanted material from the manufacturing process.
The criticality of the quality attributes can be prioritized as very high through very low based on their known impact to clinical outcomes, including both safety and efficacy. For those that are very high, the biosimilar must match the observed range of the reference product. Statistical analyses are often conducted on important quality attributes to ensure that the assessment of equivalence is rigorous and impartial.
Comparative quality attributes that are of low or very low clinical importance can be conducted in a descriptive manner without statistical analyses. Differences between the biosimilar and reference product that are not critical are permissible, as long as it is known that the differences are clearly known not to be clinically important.
Biosimilarity is established when the totality of evidence supporting a biosimilar is assembled through analysis of all public information, testing of the reference product, and the stepwise comparative development of the biosimilar and reference product.
Once satisfied that the totality of evidence is both substantial and sensitive enough to detect potential differences and to address residual uncertainty that may exist, the FDA can approve the biosimilar.
The biosimilar regulatory pathway specifies that a biosimilar is approvable for use in one or more indications on the basis of the biosimilar being highly similar to the reference product, notwithstanding minor differences that may exist in clinically inactive components, and that there are no clinically meaningful differences between the biosimilar and the reference product in terms of safety, purity, and potency.
This means that patients and their healthcare providers can be confident that the biosimilar will provide the same clinical outcomes as the reference product.
Extrapolation is the approval of a biosimilar for one or more conditions of use (known as “indications and usage”) for which its reference biological product is licensed but for which there may not be a separate clinical study for each approved indication. This approval is based on rigorous scientific evaluation of the existing data from the reference product, as well as the totality of all data (e.g., comparative analytical, functional, pharmacokinetic and pharmacodynamic, immunogenicity, and if conducted, clinical studies) obtained with the biosimilar in direct comparison to the reference product when it is known to the extent possible that the molecule works in the same way in both the studied and extrapolated indication.
There must also be no differences in mechanism of action, toxicity, or immunogenicity across indications. It is important to appreciate that extrapolation is based on the knowledge of the reference product and the totality of evidence obtained with the biosimilar molecule, and is not based on the clinical similarity of two or more different indications.
The FDA examines requests for extrapolation on a case-by-case basis. Every indication for which extrapolation is sought must be scientifically justified by the manufacturer seeking extrapolation for their product. Approval of an additional indication through scientifically valid extrapolation expedites the development of biosimilars and eliminates unnecessary clinical studies, thereby increasing patient access to important therapies.
Approval of an additional indication through scientifically valid extrapolation expedites the development of biosimilars and increases patient access to important therapies.
Scientific justification for extrapolation should address, for example, the following issues for the tested and extrapolated conditions of

The requirements to obtain an “interchangeabity” designation in the U.S. are separate from the requirements to be designated as “biosimilar.” FDA’s approval of an interchangeable biosimilar does not indicate a higher standard of biosimilarity, but that it underwent further clinical evaluation to allow it to be substituted for the reference product without first consulting the health care provider that wrote the prescription; subject to state law. Interchangeability is a secondary level of regulatory designation unique to the U.S. and is not a quality designation.
Interchangeable biosimilars are also called interchangeable biologics – the two terms mean the same thing.
According to U.S. law, an interchangeable biologic is a distinct category of biosimilar for which additional data is provided and is not a different product. To obtain an FDA designation of “interchangeability,” the manufacturer of a biosimilar typically must conduct an additional clinical study with multiple switches back and forth between the biosimilar and reference product. This clinical study must prove that for a product administered to an individual more than once, the risk in terms of safety or diminished efficacy of switching back and forth between the proposed interchangeable biologic and its reference product is not greater than the risk of using the reference product by itself, without switching. It must be established that the interchangeable biologic can be expected to have the same clinical result in all patients and indications for which the reference product is approved.
There is no legal or regulatory requirement that suggests or requires that a biosimilar meet the statutory definition of interchangeability as a prerequisite for a physician to switch a patient from a reference product to a biosimilar. Physicians always have the freedom to prescribe whatever drug they believe is appropriate for their patients.
There are no additional quality requirements to establish interchangeability, as the additional data required is clinical in nature. In fact, the interchangeable biologic product is the same biosimilar product that was initially studied, but for which additional clinical information is provided to address the specific regulatory requirements.
After a product is approved as either a biosimilar or an interchangeable biologic, the FDA will publish this information in an online database called the Purple Book. The Purple Book is updated when the FDA licenses a biological product under section 351(a) (an original new biological drug) or section 351(k) (a biosimilar or interchangeable biologic) of the Public Health Service Act (PHSA). The Purple Book identifies the date of first licensure for biological product under section 351(a) and also specifies if a 351(k) biologic is a biosimilar or an interchangeable biosimilar. Health care professionals can check the Purple Book to see if a product is a biosimilar or an interchangeable biosimilar in a manner comparable to the way that the Orange Book is used to check if the FDA has found a generic drug to be bioequivalent to its reference product.
While the FDA will designate biosimilar interchangeability, U.S. states regulate the practice of pharmacy, including laws describing how and when a pharmacist can substitute one drug for another. State substitution laws permitting pharmacy-level substitution of an interchangeable biosimilar for a reference product are very similar from state-to-state, but there are some differences. Details of individual state substitution laws can be located here.